Abstract
[Introduction]
Despite the improvement in the treatment for childhood acute lymphoblastic leukemia (ALL), a central nervous system (CNS) involvement is still a risk factor for mortality. Moreover, CNS-directed treatments can cause acute and late adverse complications. Therefore, more effective and less toxic treatment strategy for CNS-ALL is needed.
Recent clinical trials show that the anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy have an excellent effect against refractory and relapsed ALL. In these trials, some clinical effect of systemic intravenous (i.v.) delivery of CAR-T cells against CNS-ALL were reported and these reports implied clinical possibility and necessity for optimization of CAR-T therapy against CNS-ALL.
The superior effect of direct delivery of CAR-T cells into solid tumors has been published, and we and others have shown the feasibility of intrathecal (IT) donor lymphocyte infusion for CNS-ALL without severe adverse events (Yanagisawa et al, IJH 2016). These reports inspired us to apply direct CAR-T cells infusion into CNS as a new therapeutic approach against CNS-ALL. However, as the lethally neurotoxicity has been reported in CAR-T clinical trials, preclinical study is a crucial step for clinical use of IT administration of CD19 CAR-T cells.
We have previously reported ALL patient-derived xenograft (PDX) model with CNS infiltration in NOD/SCID/γc Null (NOG) mouse (Kato et al, ASH 2010). With this model, we have revealed the unique characteristics of CNS infiltrated leukemic cells and proposed new strategy targeting the CNS-ALL (Kato et al, Blood, 2018).
In this study, we established an isolated CNS-ALL xenograft model with the intra-cerebroventricular (i.c.v.) injection of ALL cells and tested the feasibility and safety of the i.c.v. delivery of CAR-T cells into this model.
[Method]
CD19 CAR-T cells were generated with the piggyBac transposon system from isolated PBMCs donated from a healthy volunteer and expanded in the presence of IL-7 and IL-15 (Morita et al, Mol Ther Methdos Clin Dev 2018). SUSR cells, BCR-ABL fusion gene positive pre-B ALL cells, were genetically engineered to express GFP-FFLuc (SUSRGFP/luc).
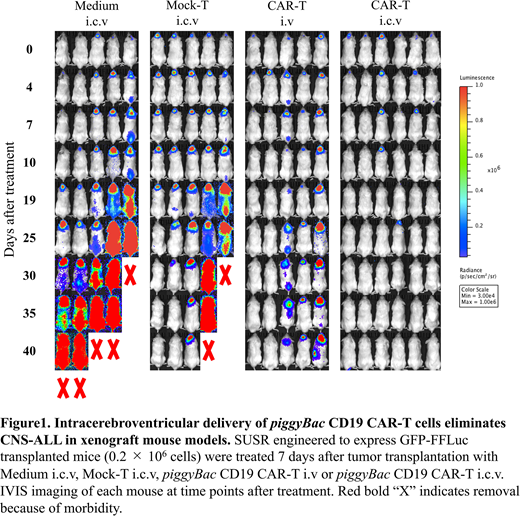
2×105 SUSRGFP/luc cells were injected by i.c.v. into 8-10 weeks old male NOG mice. Seven days after injection, confirming that SUSRGFP/luc cells were detected by IVIS imaging system only in CNS region, 2×106 piggyBac CD19 CAR-T cells injected by i.c.v. (CAR-T i.c.v.) (n=6), or i.v via tail-vein (CAR-T i.v) (n=5). As control groups, non-transduced T-cells from the same donor (Mock-T i.c.v.) (n=5) or vehicle (Medium i.c.v.) (n=5) were injected by i.c.v. No treatment group (n=4) received no i.c.v. injection. ALL invasion was followed by IVIS imaging. To assess the safety, we serially measured body weight, rectal temperature and scores of clinical symptoms of each mouse every morning after treatment.
[Results]
All mice in the Medium i.c.v. and no treatment group died by day 45. Both in the CAR-T i.v. and i.c.v. group, all mice were alive at day 45. Regardless of the route of injection, CAR-T administration prolonged the survival period. IVIS imaging revealed that 3/5 mice both in Mock-T i.c.v. and CAR-T i.v group failed to eliminate CNS-ALL at day 40. On the other hand, CAR-T i.c.v. completely eradicated ALL in all mice. Histopathological and flowcytometry analysis at day 4 after treatment also showed decrease of ALL cells and expansion of human CD3 positive T-cells in CAR-T i.c.v. mouse.
Regarding clinical symptoms, weight loss, decrease in rectal temperature and elevation of clinical scores were observed only in SUSR transplanted CAR-T i.c.v. group during a few days after treatment. These changes were transient and not fatal. No neurological symptoms were observed in all mice. Histopathological analysis of CNS at day 4 showed slightly thickened epithelium of choroid plexus and edema localized around the lateral ventricle only in CAR-T i.c.v. mouse. Any other abnormalities associated neurotoxicity was not detected.
[Discussion]
In this preclinical study, we demonstrated that i.c.v. delivery of CD19 CAR-T cells had superior effect to i.v. delivery without any additional side effects. Our data suggests that IT administration of CD19 CAR-T cells would be an effective and feasible therapeutic approach against CNS-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.